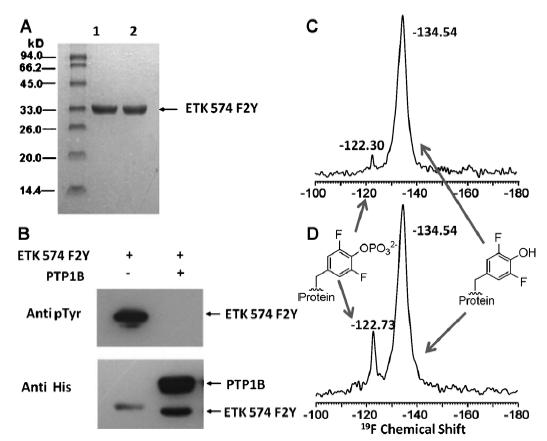
Recently, a new protein tyrosine phosphorylation quanlitative and quantitative analysis method was developed using 19F labeled unnatural amino acids with solid state NMR by scientists in Chinese Academy of Sciences.
Using the 19F solid state NMR methods, tyrosine phosphorylation levels of E. coli tyrosine kinases, Homo sapiens tyrosine kinase src (a vital enzyme in oncogenesis) were detected. And inhibition interaction of anti-cancer drug dasatinib against Src was also analyzed, which will provide a good reference and basis for further inhibitive drugs design or optimization against tyrosine kinases.
The 19F NMR experiments were conducted by double adjunct Prof. Changlin Tian of High Magnetic Field Laboratory, Chinese Academy of Sciences (CHMFL) and School of Life Sciences, University of Science and Technology of China (USTC) collaborating with Prof. Jiangyun Wang, Prof. Weiming Gong in Institute of Biophysics, Chinese Academy of Sciences on a 400 MHz wide bore solid state NMR spectrometer in USTC and the 600 MHz wide bore solid state NMR spectrometer in CHMFL
This paper has been reported online in Angewandte Chemie International Edition on Feb. 28th, 2013, titled “A Genetically Encoded 19F NMR Probe for Tyrosine Phosphorylation”
( http://onlinelibrary.wiley.com/doi/10.1002/ange.201300463/abstract ).
E. coli Tyrosine Phosphorylation Detection using 19F Nuclear Magnetic Resonance
New Protein Tyrosine Phosphorylation Qualitative and Quantitative Analysis Method Found
contact:
Dr. SHI Pan
High Magnetic Field Laboratory, Chinese Academy of Sciences
Email:shipan@hmfl.ac.cn