Apr 23,2013|By
According to an letter published online December 2012 in Nature (doi:10.1038/nature11699), Dr. WANG Junfeng’s group from High Magnetic Field Laboratory, Hefei Institutes of Physical Science, Chinese Academy of Sciences and their colaborators, reported a positive feedback regulation mechanism which amplifies and sustains T-cell receptor activation and should enhance T-cell sensitivity to foreign antigens.
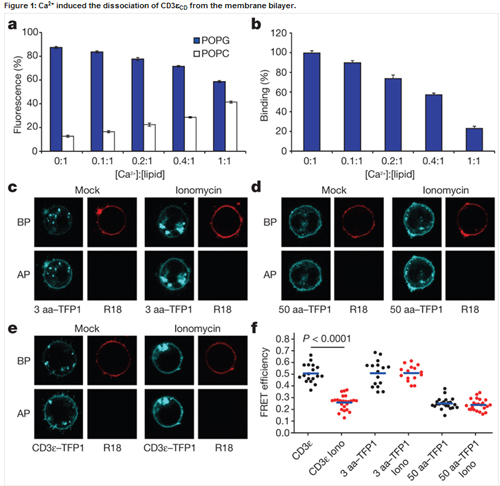
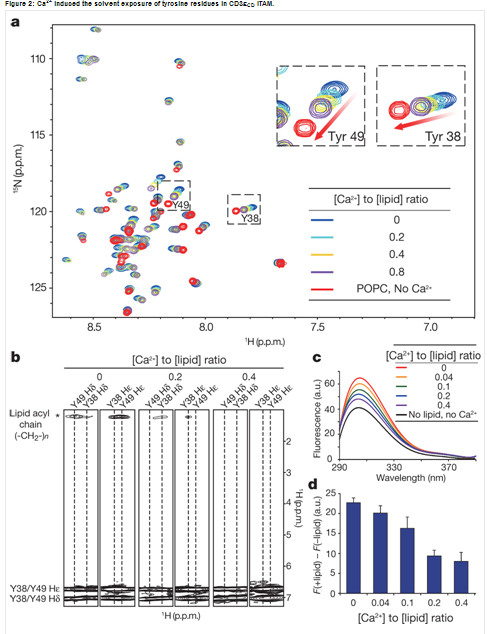
T cell signal pathway is almost clear in cytoplasm. But how the antigenic-stimulated signal transmits across the membrane remains largely unknown. Given the fact that local Ca2+ concentration of the plasma membrane rises quickly in few seconds after initial TCR triggering, this study shows that membrane-proximal Ca2+ can bind to the phosphate group in anionic phospholipids and neutralize their negative charges, which results in the dissociation of CD3 cytoplasmic domains from the membrane and the solvent exposure of tyrosine residues. As a consequence, CD3 tyrosine phosphorylation is significantly enhanced by Ca2+ influx.
Membrane binding proteins are a group of proteins that adhere temporarily to biological membranes. The reversible attachment of these molecules to biological membranes make them ideal participants in cell signaling regulation and many other important cellular events. However, structural studies on membrane proteins lag far behind than those on soluble proteins, and this, in large part, is due to technical difficulties in preparing homogeneous, stable and structurally relevant samples in a membrane-like environment. Phospholipid bilayer Nanodiscs are novel model membranes and have proven to be useful in structural studies on membrane proteins. NMR is a powerful tool to determine the solution structures and characterize the interactions between macromolecules, especially the weak interactions. Combining NMR with Nanodiscs, the Wang lab makes great progress in studying the interactions between membrane binding proteins and membrane.
This work was colaborated with Dr. XU Chenqi from Institute of Biochemistry and Cell Biology, SIBS, CAS and Dr. LIU Wanli from Tsinghua University. This work was funded by Chinese Academy of Sciences (Hundred Talents Program), National Basic Research Program of China and National Natural Science Foundation of China.
Contact:Dr. WANG Junfeng
High Magnetic Field Laboratory,Chinese Academy of Sciences
Email:junfeng@hmfl.ac.cn
Relative Link:http://www.nature.com/nature/journal/vaop/ncurrent/full/nature11699.html
Attachments Download: