Professor Yanhui XU from Fudan University and their collaborators reported that they determined the crystal structures of an important DNA methyltransferases DNMT3A in complex with its partner DNMT3L and/or histone H3 in an autoinhibitory form and active form. Dr. Pan SHI from High magnetic field laboratory, Chinese Academy of Sciences (Changlin TIAN’s Group) analyzed the conformational change of DNMT3A when bound to different methylated H3 histone. Together, the study provides a new insight into an unexpected autoinhibition and histone H3-induced activation of the de novo DNA methyltransferase after its initial genomic positioning.
DNA methylation is an important epigenetic modification that is essential for various developmental processes through regulating gene expression, genomic imprinting, and epigenetic inheritance. Mammalian genomic DNA methylation is established during embryogenesis by de novo DNA methyltransferases, DNMT3A and DNMT3B, and the methylation patterns vary with developmental stages and cell types. Structural and biochemical analyses indicate that the enzyme is mainly activated at proper targeting loci when unmethylated H3K4 is present, and strongly supports a negative correlation between H3K4me3 and DNA methylation across the mammalian genome.
NMR is a powerful tool in studying conformational change and dynamics of proteins. Site-specific 19F labeling using unnatural amino acid is successfully used to analyze the conformational change of large protein complex by Changlin TIAN’s group. The conformation of DNMT3A when bound to methylated/unmethylated histone H3 was analyzed by Dr. Pan SHI using this method. The results showed that DNMT3A can only be regulated by unmethylated histone H3.
The research results entitled "Structural insight into autoinhibition and histone H3-induced activation of DNMT3A" was accepted by Nature and published online at Nov 10th, 2014 (doi: 10.1038/nature13899).
The online link of this article:
http://www.nature.com/nature/journal/vaop/ncurrent/full/nature13899.html
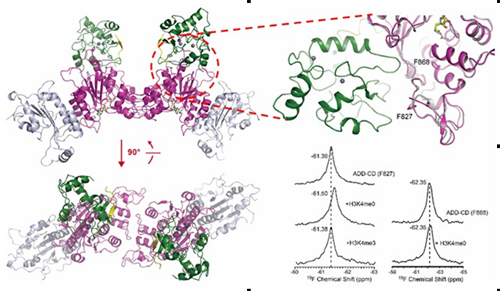 |
|
One-dimensional 19F NMR measurements were performed using ADD–CD with substitution of F827tfmF or F868tfmF in the absence or presence of H3K4me0 or H3K4me3 peptide. |
