The sorption-desorption, oxidation-redox, complexation- migration, dissolution-precipitation and assimilation-bioaccumulation processes of heavy metal ions in the natural environmental system is one of the basic research content of environmental pollution chemistry. It is crucial to explicitly understand the speciation, migration, transport, bioavailability and ecotoxicity of toxic heavy metal ions in the multicomponent environmental systems.
By using the electron paramagnetic resonance (EPR) testing platform in the Steady High Magnet Field Facility (SHMFF), new research progress on the competitive sorption mechanisms of Cu(II) and Ni(II) on the natural montmorillonite mineral is achieved by the environmental chemistry team of School for Radiological and Interdisciplinary Sciences, Soochow University.
In view of the foregoing environmental background, the research team investigated the competitive sorption and selectivity sequence of Cu(II) and Ni(II) at the montmorillonite/water interface by combining the batch experiments, X-ray diffraction (XRD), EPR spectral analysis, surface complexation modeling and X-ray Absorption Spectroscopy (XAS) technique.
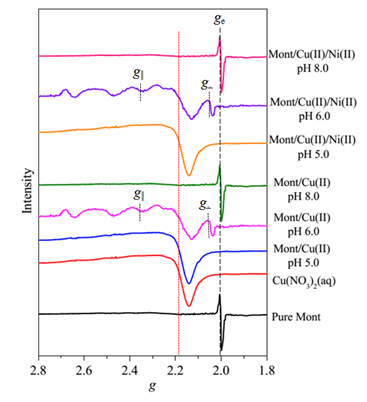
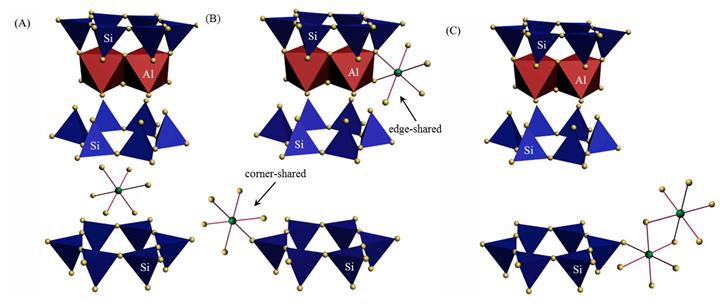
The EPR analysis results indicate that the adsorbed Cu(II) species on montmorillonite clearly change in the coordination environment with increasing pH values. Specifically, the EPR spectrum of the montmorillonite/Cu(II) sorption sample prepared at pH 5.0 appears similar to that of the Cu(NO3)2(aq) reference sample (Figure 1). This phenomenon suggests that the Cu(II) ions are adsorbed by entering the montmorillonite interlayer, forming outer-sphere complexes (Figure 2A). The spectrum of the sample prepared at pH 6.0 exhibits a distinct anisotropic rigid-limit at g values of ~2.07 and ~2.35 (Figure 1), which provides strong evidence for the occurrence of inner-sphere complexation (Figure 2B) with higher thermodynamic stability than the outer-sphere complexes (Figure 2A). For the sorption sample prepared at pH 8.0, the complete loss of the Cu(II)-induced resonance features in the EPR spectrum (Figure 1) points to the formation of the multinuclear Cu(II) surface complexes (Figure 2C). The EPR results herein are well consistent with those deduced from the XAS analysis. The derived findings could provide significant information for precise evaluation of the fate of the coexisting heavy metal ions in multicomponent environmental systems.
Based on the fruitful research foundation and superior testing platform, the researchers are making unflagging efforts to further explore novel analytical approaches and characterization techniques for clear prediction of the speciation and fate of various pollutants in the real aquatic systems. Undoubtedly, continuous studies on these major scientific issues would further promote the fast and steady development of environmental chemistry.
The above findings have been published in the top journal of environmental geochemistry named Geochimica et Cosmochimica Acta (2015, 166, 129-145).
Link to the article:Competitive sorption and selective sequence of Cu(II) and Ni(II) on montmorillonite: Batch, modeling, EPR and XAS studies
|
|
|
Figure 1. EPR spectra of montmorillonite, Cu(NO3)2(aq) reference sample and Cu(II)-containing sorption samples. |
 |
|
Figure 2 Schematic illustrations of the possible chemical speciation for Cu(II) and Ni(II) binding on montmorillonite: (A) outer-sphere complexes; (B) inner-sphere complexes; (C) multinuclear surface complexes. |
