Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal white blood cells that accumulate in the bone marrow and interfere with the production of normal blood cells. AML is the most common acute leukemia affecting adults, and its incidence increases with age. AML still remains a serious unmet medical need. Besides standard chemotherapy, there is no targeted therapy approved in the clinic to date despite several inhibitors that are presently under clinical investigation.
FLT3 is a proto-oncogene which belongs to the receptor tyrosine kinase family. The leukemic cells of about 30% of AML patients harbor the FLT3-ITD mutation, making it one of the most frequent mutations of AML.
Ibrutinib (PCI-32765) is an irreversible BTK (Bruton’s tyrosine kinase) kinase inhibitor that has been extensively used as a tool compound to validate BTK kinase roles in B-Cell receptor-mediated signaling in B-Cell related malignances. Ibrutinib has demonstrated efficacy to block the proliferation of Diffuse Large B-Cell Lymphoma (DLBCL), Mantle Cell Lymphoma (MCL), Chronic Lymphocytic Leukemia (CLL) and Multiple Myeloma (MM) by blocking BTK kinase activity in the preclinical studies and recently was approved for the clinical application on MCL and CLL.
Recently, Prof. Qingsong Liu's group, in collaboration with Jing Liu 's group in the High Magnetic Field Laboratory, Chinese Academy of Sciences (CHMFL) reported that Ibrutinib could be a candidate drug for FLT3-ITD mutant AML cancer. The researchers observed that ibrutinib exhibited anti-proliferation activity against FLT3-ITD mutant AML cell but not WT-expressing AML cells by using kinase target-based whole cell screening library. In a series of in vitro biochemical assays, this compound potently inhibited FLT3-ITD auto-phosphorylation and downstream targets in FLT3-ITD mutant AML cells, also could arrest cell cycle in G0/G1 phase and induced apoptotic cell death. The inhibitory effect of ibrutinib on those cells was independent of the original target -BTK kinase.
Currently, Ibrutinib is under extensive clinical investigation against a variety of different B-Cell malignancies. Given the fact that the safety profile of Ibrutinib is tolerated in the patients, results of current study might help to expand the application of this drug to AML patients harboring the FLT3-ITD mutant. This is another example of personalized targeted therapy in this precision medicine era. The research group has applied for Chinese patent and international patent protection, and licensed to Anhui New Star Pharmaceutical Development Co. for collaborative development of this drug’s new application.
The research was supported by China “Thousand Talents Program”, “Hundred Talents Program” of The Chinese Academy of Sciences, Anhui Province Natural Science Foundation Annual Key Program, et al.
This work was published online in the journal: Leukemia (doi:10.1038/leu.2015.175), entitled “Ibrutinib selectively targets FLT3-ITD in mutant FLT3-positive AML”.
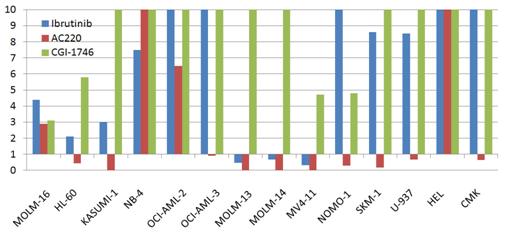 |
|
Ibrutinib anti-proliferation efficacy against AML cancer cell lines
|
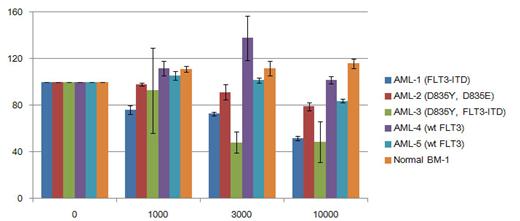 |
|
Ibrutinib anti-proliferation effect on FLT3 wt and FLT3-ITD+ patient primary cells |