Oct 16,2017|By
A joint team led by Prof. WANG Junfeng at High Magnetic Field Laboratory, Chinese Academy of Sciences (CHMFL), together with Prof. HUANG Yun and Prof. ZHOU Yubin at Texas A&M University developed novel optogenetic tools to control protein subcellular localization and interorganellar contact sites. Their work was highlighted as cover story in Chemical Science.
Endoplasmic reticulum (ER) forms an extensive intracellular membranous network in eukaryotes that dynamically connects and communicates with diverse subcellular compartments such as plasma membrane (PM) through membrane contact sites (MCSs), with the inter-membrane gaps separated by a distance of 10–40 nm.
Phosphoinositides (PI) constitute an important class of cell membrane phospholipids shared by many MCSs to regulate a myriad of cellular events, including membrane trafficking, calcium homeostasis and lipid metabolism.
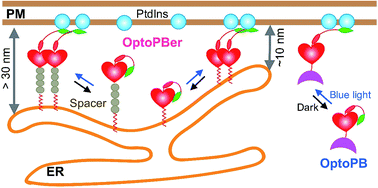
The joint team developed a genetically-encoded, single-component optogenetic tool (designated as 'OptoPB') to enable rapid and reversible control of protein translocation and inter-membrane tethering at MCSs.
To achieve optical control over protein–phospholipid interactions with least perturbation to the host cells, a small genetically encoded photoswitch – the LOV2 domain was fused to phospholipid-binding PB domains.
NMR and molecular dynamics simulation showed in dark state, the poly-basic Rit-PB docked toward two negatively charged pockets within LOV2. Once expressed in the cytosol, OptoPB can be exploited as a single component modular scaffold to deliver proteins of interest toward PM upon light stimulation.
Next, OptoPB was tethered toward the cytosolic side of ER membrane to manipulate the formation of MCS between ER and PM. To tune the gap distances at nanometer scales between ER and PM, two to eight alpha-helical spacers composed of were added.
The blue light-induced exclusion of YFP-ORAI1 from the OptoPBer-labelled membrane contact sites was gradually alleviated after the insertion of two, four, or eight helical spacers ((EAAAR)4 ), with the calculated intermembrane separation distances in the range of ~ 15–35nm.
This study provides an optogenetic toolkit that can be widely applied to aid the study of protein–lipid interactions in cellulo, to expedite the mechanistic dissection of membrane contact sites in situ, and to enable remote control of intermembrane communications occurring at these specialized subcellular structures.
The similar engineering strategies can be broadly extended to photo-manipulate other types of interorganellar contact sites, such as the ER-endosome/lysosome, ER-peroxisome, and ER-mitochondria MCSs, thus exerting remote control over cellular functions in a light-dependent manner.
This work was supported by the Natural Science Foundation of China, Ministry of Science and Technology of China and the China Scholarship Council.
|
|
|
The cover of Chem. Sci., 2017,8. It shows optoPB binds to PM upon blue light stimulation. |
 |
|
The design and applications of OptoPB and OptoPBer |
Attachments Download: