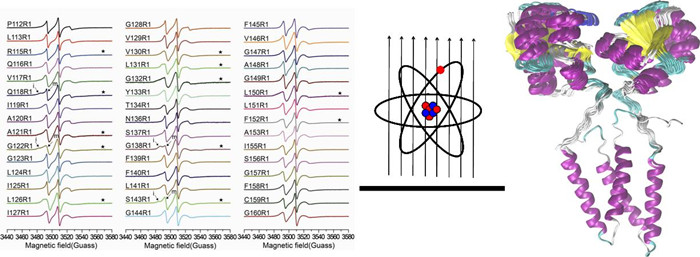
Besides X-ray crystallography, NMR, and cryo-EM, the electron paramagnetic resonance (EPR) based methods can also be applied to structural studies of integral membrane proteins.
Recently, using combinational continuous-wave EPR (CW-EPR), pulsed double electron-electron resonance EPR (DEER-EPR) and rigid-body computational methods, a joint research team determined the three-dimensional structure of YgaP, the E.coli integral membrane sulfurtransferase. This is the first de novo report of membrane protein structure determination using the up-to-date pulsed EPR method, together with CW-EPR and the rigid-body computation, despite that there are a couple of structural studies of model proteins with known structures.
Specifically, the systematic site-directed spin labeling (SDSL) was applied to the transmembrane domain of YgaP. The EPR dynamic and accessibility analysis of the YgaP variants were used to derive the secondary structure and lipid immersion of the YgaP-TMD. Distance restraints were obtained using combined CW-EPR and DEER methods. The secondary structural information and distance restraints were applied for rigid-body structural computations of the dimeric YgaP-TMD. Besides, long-distance restraints between the cytosolic rhodanese domain and YgaP-TMD were collected using the DEER method. Then, in conjunction with the solution NMR structure of cytosolic rhodanese domain of YgaP, which was determined in our group previously, the three dimensional structure of the full-length YgaP was accomplished.
Moreover, dynamic, accessibility and distance analyses were also applied to YgaP in the presence or absence of the enzymatic product SCN-. Structural transitions upon SCN- binding were derived, providing insights for the thiocyanate exportation mechanism of YgaP across the E. coli membrane.
The above study was done by Prof. Changlin Tian's group from High Magnetic Field Laboratory, Chinese Academy of Sciences (CHMFL), in collaboration with Dr. Likai Song's group in National High Magnetic Field Laboratory (NHMFL), Florida State University and Prof. Zhiyong Zhang in University of Science and Technology of China (USTC).
The findings have been published online in Scientific reports (Sci. Rep. 6, 20025; doi: 10.1038/srep20025 (2016)),entitled "Structure of a full-length E. coli integral membrane sulfurtransferase and its structural transition upon SCN- binding defined by an EPR-based hybrid method".
This work was conducted using the EPR instruments in High Magnetic Field Laboratory, Chinese Academy of Sciences (Hefei) and National High Magnetic Field Laboratory (Tallahassee, Florida). The work was supported by National Natural Science Foundation of China, National Key Science Research Grant of Ministry of Science and Technology, China.