Oct 16,2017|By
Recently, a research group led by Prof. CHEN Qianwang at University of Science and Technology of China (USTC) and High Magnetic Field Laboratory, Chinese Academy of Sciences (CHMFL) reported that Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene can be employed as a high-efficient and stable electrocatalyst for hydrogen evolution reaction (HER).
Hydrogen energy has been intensely investigated as an ideal alternative to the conservative fossil fuels for its high gravimetric energy density, zero-emission and earth-abundance.
The scalable production of hydrogen could conveniently be realized by alkaline water electrolysis. Unfortunately, water splitting is not favored kinetically and hence requires highly active electrocatalysts to be expedited.
Currently, the scalable hydrogen production is still difficult to be commercialized via electrocatalytic water splitting, limited by the lack of active and cheap hydrogen evolution electrocatalysts in basic media.
CHEN's group has made progress in electrochemical hydrogen evolution electrocatalysts in basic media.
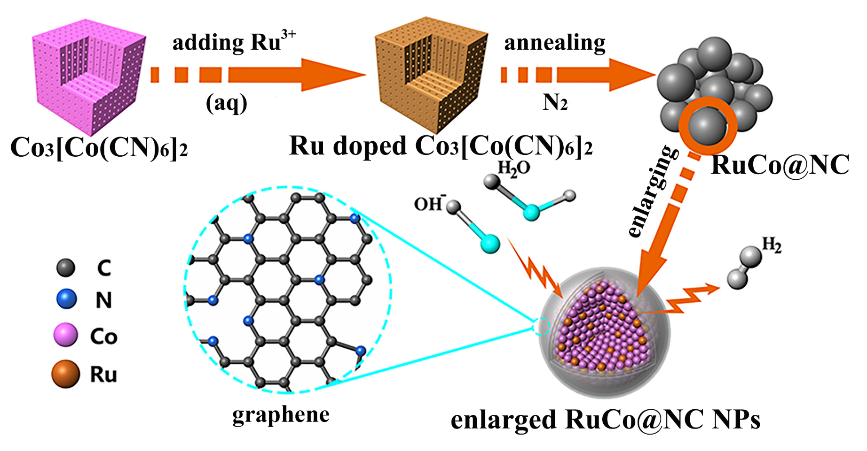
By using Ru-doped Prussian blue analogues nanoparticles as precursors, they adopted a one-step thermal decomposition strategy to fabricate high-efficient and stable electrocatalysts that was composed of ruthenium and cobalt bimetallic nanoalloy encapsulated in nitrogen-doped graphene layers.
According to the study, the catalysts display remarkable performance with low overpotentials of only 28 mV and 218 mV at 10 mA cm-2 and 100 mA cm-2, respectively, and excellent stability of 10 000 cycles. Ruthenium is the cheapest Platinum group metal and its amount in the catalyst is only 3.58 wt.%, showing the catalyst high activity at a very competitive price.
Density functional theory calculations reveal that the introduction of ruthenium atoms into cobalt core can improve the efficiency of electron transfer from alloy core to graphene shell, which is beneficial for enhancing carbon-hydrogen bond, thereby lowering GH* of HER.
The work provides a new way for the development of high-performance HER electrocatalysts in alkaline media while reducing the cost of noble metal electrocatalysts.
This work was recently published in Nature Communications entitled Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media.
This work was supported by National Natural Science Foundation, CAS/ SAFEA International Partnership Program, and Fundamental Research Funds for the Central Universities.
 |
|
Schematic illustration of the synthetic route and model of the RuConanoalloys encapsulated in nitrogen-doped graphene layers |
Attachments Download: