Apr 08,2014|By
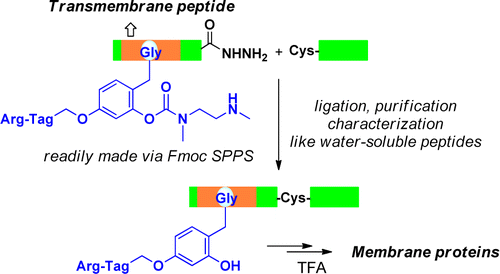
Total chemical synthesis provides a unique approach for the access to uncontaminated, monodisperse, and more importantly, post-translationally modified membrane proteins. In the present study we report a practical procedure for expedient and cost-effective synthesis of small to medium-sized membrane proteins in multimilligram scale through the use of automated Fmoc chemistry. The key finding of our study is that after the attachment of a removable arginine-tagged backbone modification group, the membrane protein segments behave almost the same as ordinary water-soluble peptides in terms of Fmoc solid-phase synthesis, ligation, purification, and mass spectrometry characterization. The efficiency and practicality of the new method is demonstrated by the successful preparation of Ser64-phosphorylated M2 proton channel from influenza A virus and the membrane-embedded domain of an inward rectifier K+ channel protein Kir5.1. Functional characterizations of these chemically synthesized membrane proteins indicate that they provide useful and otherwise-difficult-to-access materials for biochemistry and biophysics studies.
Related inks to this article:http://http://pubs.acs.org/doi/abs/10.1021/ja500222u
Attachments Download: